The Council for Scientific and Industrial Research (CSIR), in collaboration with a number of local partners, has completed work on a local ventilator to be rolled out nationwide to patients showing respiratory distress in the early phase of Covid-19 infection.
The development forms part of government’s National Ventilator Project (NVP), which falls under the auspices of the Department of Trade, Industry and Competition (DTIC), and is supported by the Solidarity Fund.
The first batch of ventilators will be provided to State hospitals around the country that are currently experiencing pressure owing to the unavailability of equipment to deal with the pandemic, says the CSIR in a statement.
Supply to the hospitals will start at the end of July or the first week of August.
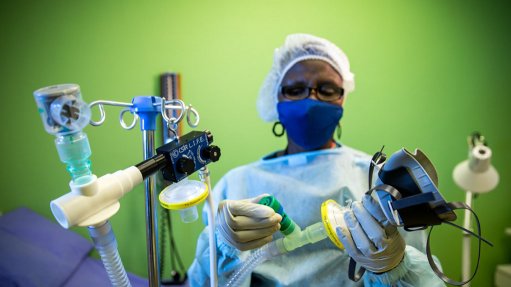
The ventilator is a continuous positive airway pressure (CPAP) device that provides a mild level of oxygenated air pressure to keep the airways open and assist with breathing.
The units are non-invasive and fill the need for readily available breathing apparatus, which are easily deployed and applied – even outside of hospitals if needs be – for intervention in cases where patients are at an early, not-intensive stage of respiratory distress, explains the CSIR.
This means the device can be used in high-tech clinical environments, as well as temporary settings, such as field hospitals and quarantine facilities that have been established across the country to handle the rising number of Covid-19 cases.
Under the project name, CSIR LIFE (Lung Inspiratory Flow Enabler), the system uses standard, hospital-grade oxygen supply, and features easy-to-use, on-device flow gauges to adjust fraction of inspired oxygen in steps of 10% oxygenation, says the CSIR.
Design and Manufacture
The device is wholly designed and produced in South Africa by the CSIR and local manufacturing and industry partners such as Siemens, Simera, Akacia, Gabler, Umoya and the University of Cape Town (UCT), with others soon to join.
The clinical requirement from the NVP was for the rapid development and distributed production of a non-invasive pre-intubation ventilation solution that could be used for most hospitalised Covid-19 patients as part of government’s response plan to the pandemic, says CSIR Future Production manufacturing executive manager Martin Sanne.
“While ensuring that we achieve this in a short period of time, we had to ensure that we follow a rigorous, documented product lifecycle methodology that would ensure scalable manufacturing, as well as compliance and licensing under the South African Health Products Regulatory Authority (SAHPRA) and [adherence to the] guidelines of the World Health Organisation.”
Siemens provided the necessary software support for the product lifecycle management, as well as software to facilitate rapid production scaling.
This included components for systems engineering processes, computer-aided design tools, manufacturing execution tools, as well as quality management solutions that would ensure compliance with health product regulations for certification.
Using a digital product lifecycle design methodology also ensures that the product can be manufactured in multiple factories in the industry and in large volumes.
“In order to ramp up capacity in a fast and efficient way, [the] CSIR has created a distributed approach to the manufacturing,” says Sanne.
“This means a number of suppliers of parts of the product have been nominated. The elements come together at a distribution hub where final quality checks, sanitisation and packaging happens before shipping the ventilators directly to hospitals and clinics.
“The partners will be named in due course. We are still in the pre-production phase and manufacturing will start from [mid-July].
“We will initially produce 10 000 units in batches by the end of August. If demand increases we will be able to further increase production during August to make more than this initially envisaged quantity.
“This way, we remain true to the role of the CSIR – which is to perform research and development (R&D) that is cutting edge, involves local industry in their niche areas, and ensures that together we address issues that are of national importance,” explains Sanne.
By June, the necessary R&D had been completed and the CPAP system was tested at UCT’s Medical Devices Laboratory, which houses specialised apparatus to evaluate such products.
This led to regulatory approval, with all the necessary licensing from SAHPRA also obtained.
There are ongoing discussions between the CSIR and the Department of Health and DTIC to produce additional devices before the end of August.
In another project, the CSIR is also working on a bi-level positive airway pressure ventilator with a local partner to develop a solution for patients with more severe symptoms.
These units assist with both inhalation and exhalation, either in fixed pressure modes or by sensing the oxygen supply required by a patient and adjusting the pressure accordingly.